二氧化碳性质表
二氧化碳及干冰的一些性质如下叙述。
基本訊息
结构与性质
| 结构与性质 | |
|---|---|
| 折射率, nD | 1.000449 at 589.3 nm and 0 °C [1] |
| 介电常数, εr | 1.60 ε0(0 °C, 50 atm) |
| C=O键平均键能 | 804.4 kJ/mol at 298 K(25 °C)[2] |
| 键长 | C=O 116 pm (1.16 Å) |
| 键角 | O-C-O: 180° |
| 磁化率 | ? |
| 表面张力 | 4.34 dyn/cm(20 °C,平衡压力) |
| 平衡压力下的液体黏度[3] | 0.0925 mPa·s at 5 °C 0.0852 mPa·s at 10 °C 0.0712 mPa·s at 20 °C 0.0625 mPa·s at 25 °C 0.0321 mPa·s at 31.1 °C |
热力学性质
| 相性质 | |
|---|---|
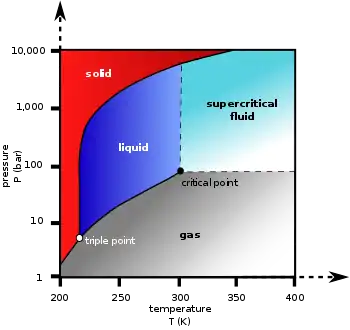
| 三相点 | 216.58 K(−56.57 °C), 518.5 kPa |
| 临界点 | 304.18 K(31.03 °C), 7.38 MPa |
| 标准熔化焓变, ΔfusH |
8.647 kJ/mol(三相点)[4] |
| 标准熔化熵变, ΔfusS |
? J/(mol·K) |
| 标准汽化焓变,[5] ΔvapH |
15.326 kJ/mol at 215.7 K(−57.5 °C) |
| 标准汽化熵变, ΔvapS |
70.8 J/(mol·K) |
| 固体性质 | |
| 标准摩尔生成焓, ΔfH |
-427.4 kJ/mol |
| 标准摩尔熵,[6] S |
51.07 J/(mol·K) |
| 热容量,[6] cp | 2.534 J/(mol·K) at 15.52 K(−257.63 °C) 47.11 J/(mol·K) at 146.48 K(−126.67 °C) 54.55 J/(mol·K) at 189.78 K(−83.37 °C) |
| 液体性质 | |
| 标准摩尔生成焓, ΔfH |
? kJ/mol |
| 标准摩尔熵, S |
? J/(mol K) |
| 热容量, cp | ? J/(mol·K) |
| 导热系数[7] | 31.0×10-5 cal/cm·s·°C(-20°C) 25.4×10-5 cal/cm·s·°C(0°C) 19.7×10-5 cal/cm·s·°C(20°C) |
| 蒸汽压[7] | 19.449 atm(-20°C) 34.397 atm(0°C) 56.525 atm(20°C)71.166(30°C) |
| 气体性质 | |
| 标准摩尔生成焓, ΔfH |
−393.52 kJ/mol |
| 标准摩尔熵, S |
213.79 J/(mol·K) |
| 热容量,[8] cp | 33.89 J/(mol K) at –75 °C 36.33 J/(mol K) at 0 °C 36.61 J/(mol K) at 15 °C 38.01 J/(mol K) at 100 °C 43.81 J/(mol K) at 400 °C 50.87 J/(mol K) at 1000 °C 56.91 J/(mol K) at 2000 °C 53.01 J/(mol K) at 38 °C, 2457 kPa 60.01 J/(mol K) at 38 °C, 5482 kPa 183.1 J/(mol K) at 38 °C, 8653 kPa |
| 绝热指数[8] γ = cp/cv |
1.37 at –75 °C 1.310 at 0 °C 1.304 at 15 °C 1.281 at 100 °C 1.235 at 400 °C 1.195 at 1000 °C 1.171 at 2000 °C |
| 范德华常数[9] | a = 363.96 L2 kPa/mol2 b = 0.04267 liter per mole |
| 与一氧化碳的化学平衡[10] CO + ½O 2 → CO 2 K = pK = log10 K |
pK = 45.0438 at T = 298.16 K pK = 25.0054 at T = 500 K pK = 16.5383 at T = 700 K pK = 11.8409 at T = 900 K pK = 8.8583 at T = 1100 K pK = 6.7989 at T = 1300 K pK = 5.2943 at T = 1500 K |
溶解度
| CO2在101.3 kPa (1 atm)分压下水中的溶解度[11] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- ‡Second column of table indicates solubility at each given temperature in volume of CO2 as it would be measured at 101.3 kPa and 0 °C per volume of water.
- The solubility is given for "pure water", i.e., water which contain only CO2. This water is going to be acidic. For example, at 25 °C the pH of 3.9 is expected (see carbonic acid). At less acidic pH values, the solubility will increase because of the pH-dependent speciation of CO2.
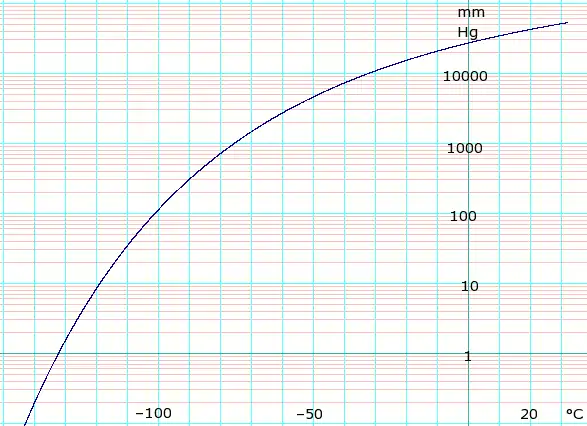
固态和气态时的蒸汽压
| P(mm Hg) | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15200 | 30400 | 45600 | |
| P(atm) (2sf,换算自mm Hg) | 0.0013 | 0.013 | 0.053 | 0.13 | 0.53 | 1.0 | 2.0 | 5.0 | 10 | 20 | 40 | 60 | |
| P(kPa)(换算自mm Hg / atm) | 0.13 | 1.3 | 5.3 | 13 | 53 | 101.325 | 202.65 | 506.625 | 1013.25 | 2026.5 | 4053 | 6079.5 | |
| T(°C) | –134.3(s) | –119.5(s) | –108.6(s) | –100.2(s) | –85.7(s) | –78.2(s) | –69.1(s) | –56.7 | –39.5 | –18.9 | 5.9 | 22.4 | |
表格数据来自《CRC Handbook of Chemistry and Physics》第44版。注释中的“(s)”表示固体上方蒸气的平衡温度。其余温度均为液体上方蒸汽的平衡温度。 For kPa values, where datum is whole numbers of atmospheres exact kPa values are given, elsewhere 2 significant figures derived from mm Hg data.
相图
液相/气相平衡热力学数据
下表列出了液相CO2在不同温度下与气相平衡的热力学数据。Heat content data, heat of vaporization, and entropy values are relative to the liquid state at 0 °C temperature and 3483 kPa pressure. To convert heat values to joules per mole values, multiply by 44.095 grams/mole. To convert densities to moles/liter, multiply by 22.678 cm3-mole/liter-gram. Data obtained from CRC Handbook of Chemistry and Physics, 44th ed. pages 2560-2561, except for critical temperature line (31.1 °C) and temperatures –30 °C and below, which are taken from Lange's Handbook of Chemistry, 10th ed. page 1463.
| 二氧化碳液-气平衡热力学数据 | ||||||||
| 温度 °C | Pvap 蒸汽压 kPa | Hliq Heat content liquid J/g | Hvap Heat content vapor J/g | ΔvapH Heat of vapor- ization J/g | ρvap Density of vapor g/cm3 | ρliq Density of liquid g/cm3 | Sliq Entropy liquid J/mol-°C | Svap Entropy vapor J/mol-°C |
|---|---|---|---|---|---|---|---|---|
| –56.6 | 518.3 | 1.179 | ||||||
| –56.0 | 531.8 | 1.177 | ||||||
| –54.0 | 579.1 | 1.169 | ||||||
| –52.0 | 629.6 | 1.162 | ||||||
| –50.0 | 683.4 | 1.155 | ||||||
| –48.0 | 740.6 | 1.147 | ||||||
| –46.0 | 801.3 | 1.139 | ||||||
| –44.0 | 865.6 | 1.131 | ||||||
| –42.0 | 933.8 | 1.124 | ||||||
| –40.0 | 1005.7 | 1.116 | ||||||
| –38.0 | 1081.9 | 1.108 | ||||||
| –36.0 | 1161.8 | 1.100 | ||||||
| –34.0 | 1246.2 | 1.092 | ||||||
| –32.0 | 1335.1 | 1.084 | ||||||
| -30.0 | 1428.6 | 1.075 | ||||||
| –28.89 | 1521 | –55.69 | 237.1 | 292.9 | 0.03846 | 1.0306 | –9.48 | 43.41 |
| –27.78 | 1575 | –53.76 | 237.3 | 291.0 | 0.03987 | 1.0276 | –9.13 | 43.21 |
| –26.67 | 1630 | –51.84 | 237.6 | 289.4 | 0.04133 | 1.0242 | –8.78 | 43.01 |
| –25.56 | 1686 | –49.87 | 237.6 | 287.5 | 0.04283 | 1.0209 | –8.45 | 42.78 |
| –24.44 | 1744 | –47.91 | 237.8 | 285.7 | 0.04440 | 1.0170 | –8.10 | 42.56 |
| –23.33 | 1804 | –45.94 | 237.8 | 283.6 | 0.04600 | 1.0132 | –7.75 | 42.36 |
| –22.22 | 1866 | –43.93 | 237.8 | 281.7 | 0.04767 | 1.0093 | –7.40 | 42.14 |
| –21.11 | 1928 | –41.92 | 237.8 | 279.6 | 0.04938 | 1.0053 | –7.05 | 41.94 |
| –20.00 | 1993 | –39.91 | 237.8 | 277.8 | 0.05116 | 1.0011 | –6.68 | 41.71 |
| –18.89 | 2059 | –37.86 | 237.8 | 275.7 | 0.05300 | 0.9968 | –6.31 | 41.49 |
| –17.78 | 2114 | –35.82 | 237.6 | 273.6 | 0.05489 | 0.9923 | –5.98 | 41.27 |
| –16.67 | 2197 | –33.73 | 237.6 | 271.2 | 0.05686 | 0.9875 | –5.61 | 41.05 |
| –15.56 | 2269 | –31.64 | 237.3 | 269.2 | 0.05888 | 0.9829 | –5.26 | 40.83 |
| –14.44 | 2343 | –29.54 | 237.3 | 266.9 | 0.06098 | 0.9782 | –4.91 | 40.61 |
| –13.33 | 2418 | –27.41 | 237.1 | 264.5 | 0.06314 | 0.9734 | –4.54 | 40.39 |
| –12.22 | 2495 | –25.27 | 236.9 | 262.2 | 0.06539 | 0.9665 | –4.17 | 40.15 |
| –11.11 | 2574 | –23.09 | 236.7 | 259.7 | 0.06771 | 0.9639 | –3.80 | 39.92 |
| –10.00 | 2654 | –20.90 | 236.4 | 257.3 | 0.07011 | 0.9592 | –3.43 | 39.68 |
| –8.89 | 2738 | –18.69 | 235.9 | 254.8 | 0.07259 | 0.9543 | –3.06 | 39.46 |
| –7.78 | 2823 | –16.45 | 235.7 | 252.2 | 0.07516 | 0.9494 | –2.69 | 39.22 |
| –6.67 | 2910 | –14.18 | 235.2 | 249.4 | 0.07783 | 0.9443 | –2.32 | 38.98 |
| –5.56 | 2999 | –11.90 | 234.8 | 246.6 | 0.08059 | 0.9393 | –1.94 | 38.74 |
| –4.44 | 3090 | –9.977 | 234.3 | 243.8 | 0.08347 | 0.9340 | –1.57 | 38.50 |
| –3.89 | 3136 | –8.410 | 234.1 | 242.4 | 0.08494 | 0.9313 | –1.37 | 38.37 |
| –2.78 | 3230 | –6.046 | 233.6 | 239.7 | 0.08797 | 0.9260 | –0.98 | 38.12 |
| –1.67 | 3327 | –3.648 | 232.9 | 236.6 | 0.09111 | 0.9206 | –0.59 | 37.88 |
| –0.56 | 3425 | –1.222 | 232.4 | 233.6 | 0.09438 | 0.9150 | –0.20 | 37.62 |
| 0.56 | 3526 | 1.234 | 231.7 | 230.5 | 0.09776 | 0.9094 | 0.20 | 37.36 |
| 1.67 | 3629 | 3.728 | 231.0 | 227.3 | 0.1013 | 0.9036 | 0.61 | 37.08 |
| 2.78 | 3735 | 6.268 | 230.4 | 224.0 | 0.1050 | 0.8975 | 1.01 | 36.83 |
| 3.89 | 3843 | 8.445 | 229.4 | 220.5 | 0.1088 | 0.8914 | 1.42 | 36.55 |
| 5.00 | 3953 | 11.46 | 228.5 | 217.0 | 0.1128 | 0.8850 | 1.83 | 36.25 |
| 6.11 | 4067 | 14.13 | 227.6 | 213.4 | 0.1169 | 0.8784 | 2.25 | 35.98 |
| 7.22 | 4182 | 16.85 | 226.5 | 209.7 | 0.1213 | 0.8716 | 2.69 | 35.68 |
| 8.33 | 4300 | 19.63 | 225.4 | 205.8 | 0.1258 | 0.8645 | 3.12 | 35.39 |
| 9.44 | 4420 | 22.46 | 224.3 | 201.8 | 0.1306 | 0.8571 | 3.56 | 35.07 |
| 10.56 | 4544 | 25.36 | 223.1 | 197.7 | 0.1355 | 0.8496 | 4.02 | 34.76 |
| 11.67 | 4670 | 28.33 | 221.8 | 193.4 | 0.1408 | 0.8418 | 4.48 | 34.45 |
| 12.78 | 4798 | 31.35 | 220.3 | 188.9 | 0.1463 | 0.8338 | 4.94 | 34.11 |
| 13.89 | 4929 | 34.49 | 218.8 | 184.3 | 0.1521 | 0.8254 | 5.42 | 33.76 |
| 15.00 | 5063 | 37.30 | 217.2 | 179.5 | 0.1583 | 0.8168 | 5.92 | 33.41 |
| 16.11 | 5200 | 41.03 | 215.1 | 174.4 | 0.1648 | 0.8076 | 6.42 | 33.02 |
| 17.22 | 5340 | 44.48 | 213.6 | 169.1 | 0.1717 | 0.7977 | 6.96 | 32.66 |
| 18.33 | 5482 | 48.03 | 211.5 | 163.5 | 0.1791 | 0.7871 | 7.49 | 32.25 |
| 19.44 | 5628 | 51.71 | 209.4 | 157.6 | 0.1869 | 0.7759 | 8.04 | 31.83 |
| 20.56 | 5776 | 55.61 | 207.0 | 151.4 | 0.1956 | 0.7639 | 8.63 | 31.38 |
| 21.67 | 5928 | 59.66 | 204.3 | 144.7 | 0.2054 | 0.7508 | 9.24 | 30.90 |
| 22.78 | 6083 | 63.97 | 201.5 | 137.5 | 0.2151 | 0.7367 | 9.89 | 30.39 |
| 23.89 | 6240 | 68.58 | 198.4 | 129.8 | 0.2263 | 0.7216 | 10.57 | 29.85 |
| 25.00 | 6401 | 73.51 | 194.8 | 121.3 | 0.2387 | 0.7058 | 11.31 | 29.24 |
| 26.11 | 6565 | 78.91 | 190.7 | 111.8 | 0.2532 | 0.6894 | 12.10 | 28.60 |
| 27.22 | 6733 | 84.94 | 186.0 | 101.1 | 0.2707 | 0.6720 | 12.99 | 27.84 |
| 28.33 | 6902 | 91.88 | 180.4 | 88.49 | 0.2923 | 0.6507 | 14.00 | 26.95 |
| 29.44 | 7081 | 100.4 | 173.1 | 72.72 | 0.3204 | 0.6209 | 15.24 | 25.85 |
| 30.00 | 7164 | 105.6 | 168.4 | 62.76 | 0.3378 | 0.5992 | 16.01 | 25.15 |
| 30.56 | 7253 | 112.3 | 162.3 | 50.04 | 0.3581 | 0.5661 | 16.99 | 24.24 |
| 31.1 | 7391 | 0.00 | 0.4641 | 0.4641 | ||||
| 温度 °C | Pvap 蒸汽压 kPa | Hliq Heat content liquid J/g | Hvap Heat content vapor J/g | ΔvapH Heat of vapor- ization J/g | ρvap Density of vapor g/cm3 | ρliq Density of liquid g/cm3 | Sliq Entropy liquid J/mol-°C | Svap Entropy vapor J/mol-°C |
光谱数据
| UV-Vis | |
|---|---|
| λmax | ? nm |
| 消光系数, ε | ? |
| IR[lower-alpha 1] | |
| 主要吸收峰 | 2350 and 667 cm−1
(4.25 and 14.99 um) |
| NMR | |
| 1H NMR | 不适用 |
| 13C NMR | 125.0[12] |
| MS | |
| 主要碎片质量 | |
化学反应方程式
| 反应物 | 反应方程式 | 反应条件 |
|---|---|---|
| 水 | CO2 + H2O ⇌ H2CO3 | 常温、常压 |
| 氧化物 | CO2 + CaO = CaCO3 CO2 + BaO = BaCO3 | |
| 氢氧化物 | CO2 + 2 NaOH = Na2CO3 + H2O CO2 + 2 KOH = K2CO3 + H2O | 固体或溶液 |
| 碳酸盐 | CO2 + H2O + Na2CO3 → 2 NaHCO3 CO2 + H2O + K2CO3 → 2 KHCO3 CO2 + H2O + Cs2CO3 → 2 CsHCO3 CO2 + H2O + CaCO3 ⇌ Ca(HCO3)2 | 溶液 |
| 铜、氧气 | 2 Cu + O2 + CO2 + H2O → Cu2(OH)2CO3(水合物) | 潮湿空气 |
| 氨 | CO2 + 2 NH3 → NH2COONH4 | 干燥反应 |
| CO2 + 2 NH3 + H2O → (NH4)2CO3[13] | 溶液 | |
| 碳 | CO2 + C ⇌ 2 CO | 高温 |
| 镁 | CO2 + 2 Mg → 2 MgO + C | 点燃 |
| 金属有机化合物 | RMgX + CO2 → RCOOH | |
| 苯酚钠 | PhONa + CO2 → Ph(OH)COONa | |
注释
- Because nitrogen and oxygen are symmetrical and carbon dioxide and water vapor are not, the air in an infrared spectrophotometer may show absorbances for CO2 and water. This is easily overcome by subtracting a blank spectrum from the experimental spectrum, and instruments are often purged with dry nitrogen as well.
参考文献
- . NPL. [7 April 2010]. (原始内容存档于2010-10-07).
- Darwent, B. deB. (1970). "Bond Dissociation Energies in Simple Molecules" Nat. Stand. Ref. Data Ser., Nat. Bur. Stand. (U.S.) 31, 52 pages.
- Lange's Handbook of Chemistry, 10th ed. pp 1669-1674
- . Air Liquide. [1 June 2007]. (原始内容存档于2016-11-23).
- (Queriable database). Chemical Engineering Research Information Center. [8 May 2007]. (原始内容存档于2007-06-03).
- W.F. Giauque and C.J. Egan, "Carbon Dioxide. The Heat Capacity and Vapor Pressure of the Solid. The Heat of Sublimation. Thermodynamic and Spectroscopic Values of the Entropy," Journal of Chemical Physics, vol. 5, pp. 45-54, 1937.
- 卢焕章. 石油化工基础数据手册. 北京: 化工出版社
- Lange's Handbook of Chemistry, 10th ed, pp 1525-1528
- Lange's Handbook of Chemistry, 10th ed, pp 1522-1524
- Lange's Handbook of Chemistry, 10th ed. pp 1573-1576
- Lange's Handbook of Chemistry, 10th ed., p 1100
- Reich, H. J. . Organic Chem Info. University of Wisconsin. [31 May 2015]. (原始内容存档于2015年3月2日).
- 碳酸铵 页面存档备份,存于. Chemical Book. [2018-1-18]
不指明时,均指标准状态。其余信息参见Wikipedia:化学信息框。
