草酸镨
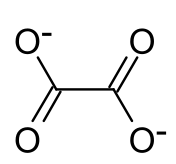
草酸镨是镨的草酸盐,化学式为Pr2(C2O4)3,存在无水物和水合物。它的十水合物受热分解得到无水物,继续加热得到氧化镨。[1]草酸镨在空气中加热分解,可以得到深棕色的十一氧化六镨。[2]它在氧气气氛中加热,能够检测到Pr(C2O4)2的生成。[3]它和盐酸反应得到Pr(C2O4)Cl·3H2O。[4]
| 草酸镨 | |||
|---|---|---|---|
 | |||
| |||
| |||
| 识别 | |||
| CAS号 | 3269-10-1 | ||
| SMILES |
| ||
| 性质 | |||
| 化学式 | Pr2(C2O4)3 | ||
| 外观 | 固体 | ||
| 若非注明,所有数据均出自一般条件(25 ℃,100 kPa)下。 | |||
参考文献
- Wendlandt, W. W. . Analytical Chemistry. 1959, 31 (3): 408–410. ISSN 0003-2700. doi:10.1021/ac60147a024.
- Sveda, Josef. The rare earths. [The oxidation of the oxalates of praseodymium and neodymium]. Casopis Ceskoslovenskeho Lekarnictva, 1927. 7. 226-232. CODEN: CCLEA3.
- Pajakoff, Swetoslaw. Tetravalent compounds of praseodymium. V. Formation of praseodymium (IV) oxalate by the thermal decomposition of praseodymium(III) oxalate in an oxygen atmosphere and application of this effect in the separation of rare earth metals. Monatshefte fuer Chemie, 1968. 99 (2): 484-500. CODEN: MOCHAP.
- Moebius, R.; Matthes, F. The exchange of oxalate ions for chloride ions of the oxalate hydrates of the rare earths and yttrium. Zeitschrift fuer Chemie, 1964. 4 (6): 234-235. ISSN: 0044-2402.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.