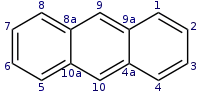
蒽
| 蒽[1] | |
|---|---|
 | |
 | |
 | |
 | |
| IUPAC名 Anthracene | |
| 识别 | |
| CAS号 | 120-12-7 |
| PubChem | 8418 |
| ChemSpider | 8111 |
| SMILES |
|
| InChI |
|
| InChIKey | MWPLVEDNUUSJAV-UHFFFAOYAK |
| Beilstein | 1905429 |
| Gmelin | 67837 |
| EINECS | 217-004-5 |
| ChEBI | 35298 |
| RTECS | CA9350000 |
| DrugBank | DB07372 |
| KEGG | C14315 |
| 性质 | |
| 化学式 | C14H10 |
| 摩尔质量 | 178.23 g·mol⁻¹ |
| 外观 | 无色 |
| 密度 | 1.25 g/cm³, 19.85℃ (固) 0.969 g/cm³, 220℃ (液) |
| 熔点 | 218 °C(491 K) |
| 沸点 | 340 °C(613 K) |
| 溶解性(其他溶剂) | 不溶于水 甲醇0.908 g/L 己烷1.64 g/L |
| 危险性 | |
| 欧盟分类 | 刺激性 對環境有害 |
| 若非注明,所有数据均出自一般条件(25 ℃,100 kPa)下。 | |
反應
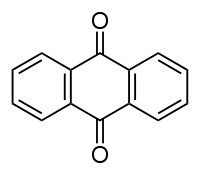 Anthraquione
Anthraquione
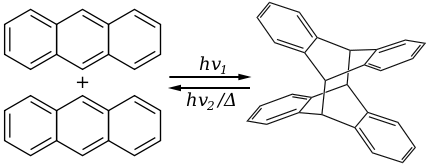
蒽亦會與親雙烯體,如單態氧進行[4+2]-環加成反應(狄耳士-阿德爾反應)。
 Diels alder reaction of anthracene with singlet oxygen
Diels alder reaction of anthracene with singlet oxygen
參見
- 菲
- 並四苯
参考资料
- NIST—蒽
- http://echa.Europa.eu/consultations/authorisation/svhc/svhc_cons_en.asp 的存檔,存档日期2011-11-18. 的存檔,存档日期2011-11-18.
- Coordination Complexes as Catalysts: The Oxidation of Anthracene by Hydrogen Peroxide in the Presence of VO(acac)2 Kimberly D. M. Charleton, Ernest M. Prokopchuk Journal of Chemical Education 2011 88 (8), 1155-1157 doi:10.1021/ed100843a
- Gerd Collin, Hartmut Höke and Jörg Talbiersky "Anthracene" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2006. doi:10.1002/14356007.a02_343.pub2
- Vatican Radio, Briefing by Fr. Federico Lombardi, 03/13/2013, 1 p.m. CET.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.